Definition from APM Body of Knowledge 7th edition. A change control system is any system that has been implemented that serves the essential purpose of assuring that the process of making changes is not done arbitrarily and without thought but rather is carefully considered and ultimately signed off on by a responsible party.
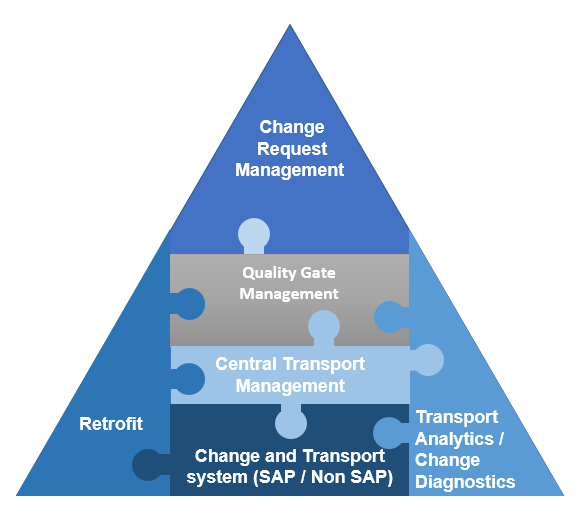 Sap Change Control Management Corealm
Sap Change Control Management Corealm
The purpose is to ensure that no unnecessary changes are made that all changes are documented that services are not unnecessarily disrupted and that resources are used efficiently.
Change control system. The change request is. As long as human and non-human components are involved in an activity or organization there are bound to be risks. Change Management is about molding hearts and minds.
Change control is a systematic approach to managing all changes made to a product or system. Risk Assessment of Change Control Generally risk assessment evaluates the potential hazards or risks involved in an activity or organization. A formal system by which qualified persons or Subject matter Expert of different departments review proposed or actual changes that might affect a validated status of facilities system equipment Document or processes.
Risk Assessment of Change Control. When Change control has to taken. Change control is the process of how changes to requirements are sourced analyzed managed and included in the roadmap and implementation schedule.
Change control is the process through which all requests to change the approved baseline of a project programme or portfolio are captured evaluated and then approved rejected or deferred. Change control is a methodology used to manage any change requests that impact the baseline of your project. That includes evaluating the request and then approving rejected or deferring it.
Change control is used to control the changes made in. Change control procedures should en- sure that sufficient supporting data are generated to demonstrate that the revised process will result in a product of the desired quality consistent with the approved specifications. 43 Change control is an important element.
A change request enters the change control system. Its a way to capture that change from the point where its been identified through every step of the project cycle. Get ready Im going to walk you through the whole darn thing now.
Regulated companies also use document change control systems to comply with change control requirements that can be found in international quality standards such as ISO 13485 Section 737 manage design and development changes and ICH Q10 Pharmaceutical Quality System Section 323 change management system. What Is Change Control. Change control is an innovative software that helps you and your organization ensure that changes are controlled and non value adding changes are stopped.
In pharmaceuticals every change is reported by change control process. When a change is being made in any process or procedure it is reported by change control procedure and that is approved by the company authority. Change Control is about governing the requirements management.
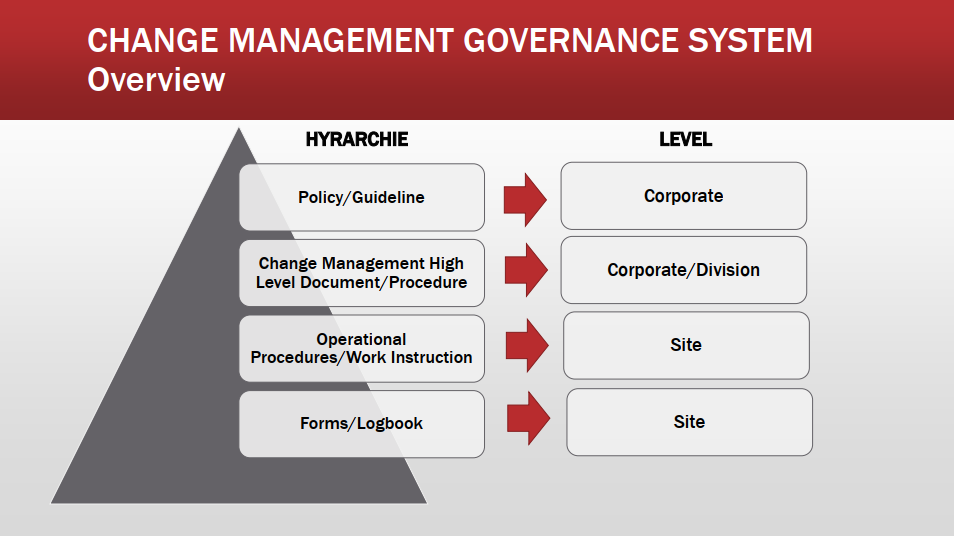
Policies work instructions and engineering changes all have to be implemented. The change control system is a collection of processes that allow change requests to be analyzed approved declined and then managed. Change management is essential in a regulated environment.
What is Change control Procedure. Change Control Systems Communicate a change across the entire organization with automatic change management.